Physics
GRIFFIN is designed for decay spectroscopy, the study of the radiation emitted when radioactive nuclei decay. Our facility supports a broad spectrum of research in three major fields: in nuclear structure, GRIFFIN helps understand how the properties of nuclei change based on their composition; in nuclear astrophysics, it studies nuclei along the r-process path (thought to be the mechanism for how the heavy chemical elements are formed in the universe); and in fundamental symmetries, it performs precision studies of super-allowed fermi beta decays in a particular subset of nuclei that have the same number of protons and neutrons, as well as measurements for the Radon Electric Dipole Moment program at ISAC.
Nuclear Structure
The atomic nucleus is a strongly-interacting, many-body quantum mechanical system that exhibits a fascinating variety of shapes and excitation modes, from spherical to super deformed (axis ratio 2:1), and from excitations of single protons and neutrons to collective vibrations and rotations of the nucleus as a whole. The study of nuclear structure attempts to elucidate the unifying mechanisms by which these rich patterns of behavior emerge from the common underlying strong nuclear interaction between the nucleons (protons and neutrons) that form the nucleus.
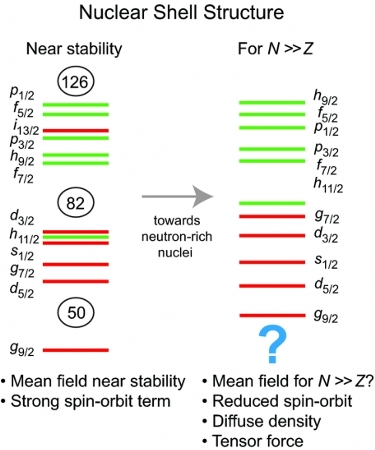
Central to these studies is the concept of nuclear shell structure, in which protons and neutrons occupy quantized energy levels within a potential generated by their interactions with all of the other nucleons. As shown on the left side of Fig. 1, these quantized energy levels cluster into groups, or shells, and the filling of major energy shells leads to particularly stable configurations associated with the proton and neutron "magic numbers": 2, 8, 20, 28, 50, 82, and 126. Nuclei near closed shells tend to have spherical ground states, while those far from shell closures are deformed and exhibit low-energy collective rotational excitations.
While the nuclear shell structure and magic numbers have been well studied close to the "valley of stability" defined by the ~ 300 stable isotopes found in nature (black pixels in Fig. 2), investigations of exotic nuclei with unusual proton-to-neutron ratios are revealing an evolution of this shell structure (right side of Fig. 1) as one moves away from the stable isotopes. Understanding of this complex evolution of nuclear shell structure is of fundamental interest both as an emergent property of the nuclear quantum many-body system and as an important input to understanding the nuclear reaction processes by which the heavy chemical elements have been, and continue to be, synthesized in explosive astrophysical environments. As shown schematically in Fig. 3, nuclear shell structure in extremely neutron-rich nuclei is thought to play a major role in the pathway for the rapid neutron capture (r) process, thought to be responsible for producing approximately half of the elements heavier than iron. Neither the exact locations of the shell gaps in these neutron-rich isotopes, nor the exact astrophysical events in which the r-process takes place, are, however, established at present.
Gamma-ray spectroscopy provides powerful techniques to study nuclear shell structure through measurements of gamma-ray energies and angular correlations in low-energy decay experiments and through measurements of electromagnetic transition matrix elements between nuclear states in Coulomb excitation experiments with accelerated radioactive ion beams. At present, the 8pi Spectrometer and its auxiliary detectors support our decay spectroscopy studies at ISAC-I, while the TIGRESS spectrometer is used in our experiments with accelerated radioactive beams at ISAC-II. We are also currently developing the state-of-the-art new GRIFFIN spectrometer that will provide 300 times higher gamma-gamma coincidence detection efficiency than the 8pi Spectrometer and revolutionize the decay spectroscopy program at ISAC-I, as well as the DESCANT neutron detector array that will be used for gamma-neutron coincidence studies in conjunction with both TIGRESS at ISAC-II and GRIFFIN at ISAC-I.
See also: University of Guelph Nuclear Physics

Superallowed Beta Decay
The process of nuclear beta decay takes place when the nucleus contains an excess of protons or neutrons relative to the combinations that form stable isotopes. Beta decay then converts a neutron into a proton, or a proton into a neutron, to achieve a more strongly bound, or stable, configuration of nucleons.
Considering the quarks substructure of the nucleons, the neutron is comprised of two "down" type quarks and one "up" type quark, while the proton is comprised of two "up" type quarks and one "down" type. As shown in Fig. 1, the beta decay process thus corresponds to the conversion of a down quark to an up quark, converting the neutron into a proton, with the emission of a virtual W- particle that decays into an electron and an anti-electron neutrino. Conversely, the conversion of an up quark in a proton to a down quark results in a neutron and a virtual W+ particle that decays to a positron and electron neutrino. These processes are governed by the weak nuclear interaction, which is unified with electromagnetism in the Standard Model of particle physics to form the electroweak force.
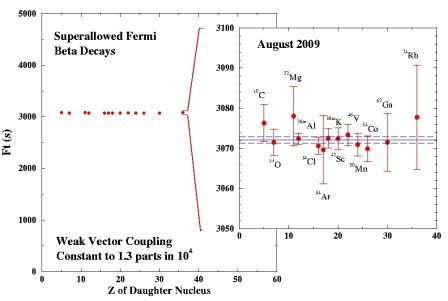
For a particular subset of nuclear beta decays, known as superallowed beta decays, the quantum-mechanical wavefunction of the entire nucleus is left essentially unchanged by the beta decay process with the exception of one proton being converted into a neutron. These special decays are particularly amenable to theoretical analysis and precise experimental measurements for superallowed beta decays thus provide demanding tests of the Standard Model description of electroweak interactions. For example, while the half-lives associated with the full range of nuclear beta decays span some 20 orders of magnitude (from thousandths of a second to billions of years), Fig. 2 shows that all 13 of the superallowed Fermi beta decays that have been measured precisely to date have identical (within their error bars) intrinsic strengths, confirming the Conserved Vector Current (CVC) hypothesis of the Standard Model at the level of 0.01% precision. These superallowed beta decay data also currently provide the most precise determination of the "up-down" element of Cabibbo-Kobayashi-Maskawa (CKM) quark mixing matrix that describes the transformation between the mass eigenstates and the weak interaction eigenstates of the Standard Model quarks.
See also: University of Guelph Nuclear Physics

Radon Electric Dipole Moment
As illustrated in Fig. 1, the orientation of an intrinsic electric dipole moment (EDM) of a particle, relative to its intrinsic angular momentum or spin, changes sign under both the parity (P) and time-reversal (T) operations. A non-zero permanent EDM for an elementary particle or atom can thus only arise from the polarization of the system by parity and time-reversal violating fundamental interactions. No such particle EDM has ever been measured, despite 5 decades of searches with ever increasing experimental sensitivity.
The global effort to perform more and more precise searches for particle EDM's is motivated by our desire to understand the fundamental symmetries of the laws of physics and the most basic origins of matter in our universe. All of the experimental evidence available to date supports the theoretically motivated expectation that all of the physical laws remain unchanged under the combined operation of CPT-reversal, where CP-reversal exchanges particles with their anti-particle partners. Assuming the validity of this CPT symmetry, the observation of a time-reversal (T) violating electric dipole moment would imply the existence of new fundamental CP-violating fundamental interactions in nature.
Additional sources of CP-violation are required to account for the observed imbalance between matter and anti-matter in our universe, as the CP-violation within the currently known Standard Model of particle physics is far too weak to do so. Many of the proposed models of physics beyond the Standard Model, such as supersymmetry (SUSY), theories with multiple Higgs bosons, and left-right symmetric models, naturally include such additional sources of CP-violation that could account for the cosmic matter/antimatter asymmetry. These CP-violating interactions also generically lead to predictions for particle electric dipole moments at, or tantalizingly close to, the current level of experimental sensitivity. EDM measurements thus provide demanding tests of physics beyond the Standard Model, and important constraints on the models of new physics required to explain the matter/antimatter asymmetry of the universe.
See also: University of Guelph Nuclear Physics

Radioactive Decay Modes
Nuclei exhibit a wide array of different decay modes, each producing a unique radioactive signal that an experiment like GRIFFIN can measure. Here we review these fundamental decays. See the glossary below for symbol and term definitions.
In this section:
β+ Decay
Process: A proton in a nucleus spontaneously turns into a neutron, a positron and an electron neutrino. The nucleus is often left in an excited state and loses energy by emitting gamma rays. β+ decay occurs in nuclei on the neutron-deficient side of the nuclear chart.
History: This process was discovered to be different to beta- decay in 1934 by Irène and Frédéric Joliot-Curie.
β- Decay
Process: A neutron spontaneously turns into a proton, an electron and an anti-electron neutrino. The daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission. β- decay occurs in nuclei on the neutron-rich side of the nuclear chart, that is nuclei containing an excess of neutrons compared to protons.
History: This process, along with alpha decay, was one of the first forms of radioactivity to be discovered by Henry Bequerel in 1896.
Electron Capture
Process: A proton in a nucleus spontaneously and simultaneously captures an atomic electron and turns into a neutron and an electron neutrino. The daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission. Electron capture competes with β+ decay on the neutron-deficient side of the nuclear chart, that is nuclei with an excess of protons to neutrons.
History: Electron capture was first theorized in 1934 by Gian-Carlo Wick, and observed in 1936-37 by Luiz Alvarez.
β-Delayed Neutron Emission
Process: A neutron inside a nucleus spontaneously turns into a proton, an electron and an anti-electron neutrino, as per β- decay, but in this case the daughter nucleus is formed in an excited state which is above the binding energy for a neutron within that nucleus so a neutron is immediately ejected. The final daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission. β-delayed neutron emission occurs in nuclei on the neutron-rich side of the nuclear chart, that is nuclei containing an excess of neutrons compared to protons.
History: Beta-delayed neutrons were first observed by studying decays of heavy fission products by Roberts, Myer and Wang in 1939.
β-Delayed Proton Emission
Process: A neutron inside a nucleus spontaneously turns into a neutron, a positron and an electron neutrino, as per β+ decay, but in this case the daughter nucleus is formed in an excited state which is above the binding energy for a proton within that nucleus so a proton is immediately ejected. The final daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission. β-delayed proton emission occurs in nuclei on the neutron-deficient side of the nuclear chart, that is nuclei containing an excess of protons compared to neutrons.
History: Beta-delayed proton emission was first observed in 1963 by Barton et al.
Double β+ Decay
Process: Two protons in a nucleus spontaneously and simultaneously turn into two neutrons, two positrons and two electron neutrinos. The nucleus is often left in an excited state and loses energy by emitting gamma rays. Double β+ decay is an extremely rare process and occurs in nuclei which appear to be stable to other decay modes.
Double β- Decay
Process: Two neutrons in a nucleus spontaneously and simultaneously turn into two protons, two electrons and two anti-electron neutrinos. The daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission. Double β- decay is an extremely rare process and occurs in nuclei which appear to be stable to other decay modes.
History: Double β decay (of either charge state) was first observed in 1987 by Elliot, Hahn and Moe.
Double Electron Capture
Process: Two protons in a nucleus spontaneously and simultaneously capture two atomic electrons and turn into two neutrons and two electron neutrinos. The daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission. Double electron capture is an extremely rare process and occurs in nuclei which appear to be stable to other decay modes.
Alpha Decay
Process: A nucleus spontaneously emits a 4He nucleus - a tightly bound system of two protons and two neutrons. The daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission or internal conversion process. Alpha decay usually occurs in heavy nuclei.
History: This process, along with beta decay, was one of the first forms of radioactivity to be discovered by Henry Bequerel in 1896.
Proton Emission
Process: A nucleus spontaneously emit a proton. The daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission. Proton radioactivity occurs in very neutron-deficient nuclei which have an odd number of protons and lie beyond the proton dripline, that means that the energy to remove a proton is less than zero. The proton is only held in the nucleus by the centrifugal barrier.
History: This process was discovered in multiple experiments at GSI in Germany in 1982.
Two-Proton Emission
Process: A nucleus spontaneously emits two protons. The daughter nucleus is often formed in an excited state and loses energy by gamma-ray emission. Two-proton radioactivity occurs in very neutron-deficient nuclei which lie beyond the proton dripline, that means that the energy to remove the two protons is less than zero. The two protons are only held in the nucleus by the centrifugal barrier.
History: This process was discovered in experiments at both GANIL and GSI in 2002 looking at the decay of 54Fe.
Cluster Radioactivity
Process: A nucleus spontaneously emits a nucleus between roughly A=14-34. Cluster radioactivity is an extremely rare process and is only exhibited by a handful of nuclei in which the daughter nucleus is close to doubly-magic, like 208Pb. In fact there are only 12 cases of cluster radioactivity discovered so far.
History: This process was discovered by Rose and Jones in 1984 when they observed that in every 890 million alpha decays of 223Ra there is one emission of a 14C nucleus instead.
Spontaneous Fission
Process: A nucleus spontaneously splits into two pieces. The fragments often release several neutrons before the final daughter nuclei are formed in an excited state and loses energy by gamma-ray emission. Spontaneous fission occurs in heavy nuclei. Fission can also be induced by bombarding a heavy nucleus with neutrons, protons or photons.
History: Spontaneous fission was first observed in 1940, by Petrzak and Flerov.
Gamma Emission
Process: A nucleus in an excited state loses energy by emitting a gamma ray. A gamma ray is a high energy form of light or photon which is essentially a packet of energy. Gamma-ray emission can occur in any nucleus on the nuclear chart. An alternative process in certain transitions is internal conversion.
History: This process was the third form of radioactivity to be discovered. It was first observed by Paul Villard in 1900.
Internal Conversion
Process: A nucleus in an excited state loses energy by passing it to an atomic electron and ejecting it from the atom. The energy of the electron is equal to the transition energy minus the binding energy of the atomic orbital from which it came. Internal conversion can occur in any nucleus on the nuclear chart but is more common in heavy nuclei above lead. An alternative process for the nucleus to lose energy is γ emission.
Glossary
- Alpha Particle, α
- An alpha particle is a bound 4He nucleus, that is, two protons and two neutrons tightly held together.
- Atom
- An atom consists of a central nucleus surrounded by a cloud of electrons.
- Electron, e- or β-
- The electron is a fundamental particle which carries one negative unit of electric charge. In an atom the electrons occupy orbitals around a central nucleus. A β- particle is an electron.
- Neutrino, νe, νμ, ντ
- A neutrino is a fundamental particle which come in three flavors; electron, muon and tau. The neutrino carries no electric charge and has almost no mass. It passes through ordinary matter with almost no effect and only very occasionally undergoes an interaction. A bar over the symbol, ν̅e, indicates an anti-neutrino, the regular neutrino's antiparticle.
- Neutron, n
- One of the components which makes up a nucleus. The neutron carries no nuclear charge. A neutron is not itself a fundamental particle as it is made up of three quarks, two down and one up quark.
- Nucleus, xNy
- A nucleus is the central part of an atom. It consists of a number of protons (x) and neutrons (y). The number of protons defines which element the nucleus is and the sum of neutrons and protons defines which isotope it is.
- Positron, e+ or β+
- The positron is a fundamental particle which is the anti-particle of the electron. The position carries one positive unit of electric charge. A β- particle is a positron.
- Proton, p
- One of the components which makes up a nucleus. The proton carries one positive unit of electric charge. A proton is not itself a fundamental particle as it is made up of three quarks, two up and one down quark.